Article Text
Abstract
Aim: Diffuse large B cell lymphoma (DLBCL) is the most common lymphoid malignancy in the western hemisphere, and is characterised by a highly variable outcome that impedes individual risk assessment. Lacking reliable biomarkers, the international prognostic index (IPI) has been the most reliable factor to predict survival and stratify patients for therapy. The aim of this study was to investigate the frequency and potential prognostic role of BCL2 aberrations on the chromosomal level and the protein level in a large DLBCL collective.
Methods: Fluorescence in situ hybridisation (FISH) with commercially available dual-colour break-apart probes and immunohistochemistry were used to assess BCL2 gene abnormalities and bcl2 protein expression on validated tissue microarrays containing 224 well-characterised cases of primary DLBCL.
Results: FISH analysis of BCL2 revealed a break in 40/215 cases (19%) and a gain in 66/171 (39%) cases. Only BCL2 gains correlated with bcl2 protein expression (p = 0.001). Presence of any BCL2 gene abnormality, particularly gains, correlated independently of the IPI with a significantly worse prognosis in DLBCL of non-germinal centre (non-GC) phenotype as opposed to DLBCL of non-GC type without this genetic alteration (p = 0.003). DLBCL of germinal centre phenotype did not show this association.
Conclusions: Cases of DLBCL of the non-GC type with BCL2 gene aberration are accompanied by a significantly worse prognosis as opposed to cases without such gene abnormalities. It may be helpful to asses BCL2 gene abnormalities by FISH in addition to assessing established parameters for individual risk estimation in DLBCL.
Statistics from Altmetric.com
Diffuse large B cell lymphoma (DLBCL) is a common lymphoid neoplasm characterised by highly variable outcomes.1 Several biological markers have been studied in attempting to identify high-risk patients, but none has proved to be completely effective,2 ,3 ,4 and the international prognostic index (IPI) has remained the gold standard for predicting prognosis.5 Gene expression profiling might offer an independent prognostic value (eg, patients with DLBCL of germinal centre (GC) B cell type tend to have a better overall survival (OS) than those with DLBCL of non-GC type6 ,7 ,8), but this technology is not yet available in routine practice. An easily applicable algorithm that identifies these subtypes based on the immunohistochemical expression pattern of CD10, bcl6, and mum1 has been developed,9 but the prognostic significance varies among the different studies.10 ,11 ,12 ,13 ,14 ,15 Thus, alternative algorithms based on the expression of bcl2 for subdividing DLBCL have been developed.11
The bcl2 protein, a mitochondrial inner-membrane protein, is known for its anti-apoptotic properties.16 It is overexpressed by many DLBCLs, and this has been associated with inferior survival in most,10 ,13 ,17 ,18 ,19 ,20 but not all studies.9 ,21 ,22 The translocation t(14;18)(q32;q21) can be detected in approximately 25% of all DLBCLs. In its absence, amplification of 18q21 can lead to overexpression of the bcl2 protein. Furthermore, gains at 18q21 may lead to overexpression of other genes, such as MALT1 and TNFRS11A (RANK), known to be frequently altered in lymphomas.23 ,24 It has been proven in animal models that excess anti-apoptotic members of the bcl2 family and lack of pro-apoptotic members contribute to lymphomagenesis and progression/persistence of DLBCL.25 ,26 DLBCL of the GC type seems to harbour t(14;18) more often than DLBCL of the non-GC type, while the latter seems to contain the amplification more frequently than the former.7 ,20 ,22 ,27 ,28 ,29 Importantly, new therapeutic approaches directed against the BCL2 gene/bcl2 protein are currently being developed (eg, small inhibitory molecules and antisense oligonucleotides).30
Based on this information, we investigated the role of BCL2 gene abnormalities and bcl2 protein expression in relation to known prognostic parameters in a large number of DLBCLs on validated tissue microarrays (TMAs).31
Materials and methods
Patients
A total of 224 cases of de novo DLBCL diagnosed between 1988 and 2000 and reclassified according to the World Health Organization criteria1 were included in this study.32 Paraffin blocks were selected on the basis of availability and preservation. Clinical and follow-up data were obtained by reviewing the charts. Retrieval of tissue and clinical data was performed according to the regulations of the local institutional review board and data safety laws. Patient characteristics are detailed in table 1.
Patient characteristics
Construction of TMAs
TMAs were constructed as described previously.31 Four different TMA contained tumour samples from different histopathological institutions (University Hospitals of Basel, Bologna, and Innsbruck, and Triemli Hospital in Zurich). Each donor tissue block was punched at least twice.
Immunohistochemistry
Sections (4 μm) of the TMA blocks were cut onto adhesive-coated slides and stained using standard procedures. The following antibodies were used: bcl2 (clone 124, Dako M0887, final dilution 1:10; Dako, Glostrup, Denmark), bcl6 (clone PG-B6p, Dako M7211, final dilution 1:10), CD10 (clone NLL-CD10-270 56C6, final dilution 1:10; Novocastra, Newcastle upon Tyne, UK), and mum1 (clone Mum-1P, Dako M7259, final dilution 1:50). For negative control experiments, species-matched and class-matched non-relevant primary antibodies were used. The slides were evaluated without knowledge of clinical data; and 20% of cases were re-evaluated by a second observer. At least 200 cells were assessed in each tumour core. Cut-off levels were determined as described elsewhere.32 ,33
Fluorescence in situ hybridisation for BCL2 gene rearrangement
Fluorescence in situ hybridisation (FISH) was performed with a locus-specific identifier BCL2 dual-colour, break-apart probe (order no. 07J75-001; Vysis, Downers Grove, Illinois, USA) on paraffin-embedded tissue sections according to the Vysis protocol. Slides were counterstained with 125 ng/ml 4′,6-diamino-2-phenylindole in antifade solution. FISH signals were scored with a Zeiss fluorescence microscope. Cases on the TMA were considered for evaluation if at least 200 tumour cell nuclei per core displayed positive signals. Abnormal FISH signals were recorded as percentage of cells showing an abnormality. The cut-off score to consider a case rearranged (“breaks”) was the mean+3SD of split nuclei in the reference cases (ie, ⩾3%). High-level 18q21 amplification was defined as the presence of either more than 10 gene signals or tight clusters of at least five gene signals. Polysomies and low-level amplifications were defined as presence of tumour cell nuclei with three or more signals exceeding the mean+3SD of tripolysome/polysome nuclei in the reference cases (ie, ⩾5%). Amplifications and polysomies were referred to as “gains”. Sections of five tonsils were used as controls. To assess reproducibility, comparison between the results gained by two observers (MC and AT) was performed in a blinded fashion in 80 cases.
Statistical analysis
Statistical analyses were performed using SPSS version 16.0 (SPSS, Chicago, Illinois, USA). Incomplete data were not excluded from the tests. The interobserver agreement for FISH was assessed using the κ statistic; a κ value of ⩾0.75 implied excellent agreement. The Spearman test was used to analyse relationships between the markers. The Kruskal–Wallis test was applied to assess mean differences between groups. Disease-specific survival (DSS) was analysed by the Kaplan–Meier method and compared by the log-rank test in univariable modus and by Cox regression analysis considering also IPI in multivariable modus. Statistical significance was defined as p<0.05 and corrected for multiple testing (adjusted p<0.017) in instances where the different phenotypic subgroups of DLBCL were separately analysed. Two-sided tests were used throughout.
Results
Patient characteristics
The mean age of patients at diagnosis was 62 years (median 66, range 12–93). There were 110 men (mean age 62 years, range 12–90), 104 women (median age 62 years, range 18–93), and 10 patients of unknown gender. Clinical follow-up data were available for 161 patients. The average follow-up time was 40 months (median 30, range 1–218) (table 1).
Immunohistochemistry
All cases were stratified into DLBCL of GC type and non-GC type according to the Hans algorithm.9 Protein expression above the cut-off scores was detected for CD10 in 52/180 evaluable cases (cut-off 7.5%), for bcl6 in 64/137 cases (cut-off 15%), and for mum1 in 42/166 cases (cut-off 65%).32 ,33 According to these cut-offs, there were 72 DLBCLs of GC type, 77 DLBCLs of non-GC type, and 75 cases that were not able to be classified because of missing data regarding the expression of either CD10, mum1 and/or bcl6. Positivity for bcl2 was found in 99/201 (49%) cases of DLBCL with a cut-off of 57.5% (DLBCL of GC type: 29/67, 43%; DLBCL of non-GC type: 41/74, 55%; p = 0.153). When cut-offs according to the original Hans algorithm were taken into account,9 there were 59 DLBCLs of GC type and 97 DLBCLs of non-GC phenotype. The percentages of bcl2-positive cases in each subgroup were almost identical regardless of the cut-offs used to stratify cases into GC type and non-GC type DLBCL.
FISH
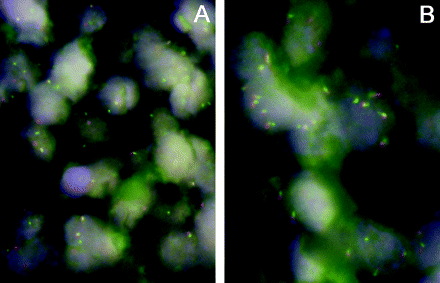
FISH results were very reproducible: κ = 1 for breaks and κ = 0.94 for gains. Fifteen of 70 cases (21%; mean (SD): 59 (38)% of cells/case) displayed breaks and 19/53 (36%; mean (SD): 29 (19)% of cells/case) displayed gains, including two with high-level amplification in DLBCL of the GC type. There were 13/75 (17%; mean (SD): 36 (33)% of cells/case) breaks and 27/64 (42%; mean (SD): 35 (28)% of cells/case) gains in DLBCL of non-GC type, including one case each with high-level amplification and double splits. There were no relevant differences in case distribution considering BCL2 aberrations when cut-offs according to the original Hans algorithm9 were taken into account (14/58 (24%) cases with breaks and 17/41 (41%) cases with gains in GC type DLBCL, and 14/93 (15%) cases with breaks and 31/82 (38%) cases with gains in non-GC type DLBCL). In the DLBCL cases that were not able to be classified, there were 12/70 (17%) breaks and 20/54 gains (37%), including one case with high-level amplification (fig 1A, B). Only one of the cases with a break showed a simultaneous gain. Neither the number of cases with breaks and/or gains nor the mean proportion of cells with genetic abnormalities was significantly different between the phenotypic subgroups. A correlation could be only found between BCL2 gains and bcl2 protein expression in the whole DLBCL collective as well as in the different phenotypic subgroups (correlation coefficient for the whole DLBCL group: 0.342, p = 0.001). There was no significant correlation between BCL2 breaks and bcl2 protein expression.
Interphase fluorescence in situ hybridisation analysis demonstrating split red and green signals corresponding to breaks (A) and increased (up to 5 per nucleus) signals corresponding to gains (B) in diffuse large B cell lymphoma cases. Dual-colour, break-apart BCL2 probe, 4′,6-diamino-2-phenylindole counterstain, ×1000.
Prognostic value of BCL2 gene abnormalities and bcl2 protein expression
All DLBCLs were analysed for possible correlation of the evaluated parameters and DSS. Clinical follow-up data were available for 149 cases with BCL2 aberrations. In the multivariable analysis, IPI remained the only significant independent prognostic predictor. However, when analysed separately for DLBCLs of GC type and non-GC type, any abnormalities of the BCL2 gene constituted a significant, IPI-independent, prognostic parameter for DSS in DLBCLs of non-GC subtype (relative risk for IPI 1.66, 95% confidence interval (CI) 1.09 to 2.53, p = 0.008; relative risk for BCL2 gene abnormalities 4.48, 95% CI 1.21 to 16.38, p = 0.017). Non-GC DLBCLs with abnormalities (11 cases with breaks, 24 cases with gains) of the BCL2 gene displayed a mean survival of 63 months (95% CI 29 to 97, median 12) with 21 lymphoma-related deaths out of 31 cases as opposed to non-GC DLBCLs without BCL2 gene abnormalities with a mean survival of 124 months (95% CI 84 to 163, median 109) and six deaths out of 24 cases (p = 0.003; fig 2). In non-GC DLBCL, this effect could be visualised by the Kaplan–Meier curves for both BCL2 breaks and gains (data not shown in detail), but it reached statistical significance only for gains (p = 0.002) versus breaks (p = 0.125). There were no relevant differences with respect to these issues when cut-offs according to the original Hans algorithm9 were taken into account. Multivariable analysis corrected for multiple testing did not prove an IPI-independence of BCL2 gains considering DSS in non-GC DLBCLs (p = 0.037). Particular consideration of high-level amplifications was of no additional prognostic value.
Disease-specific survival of patients with diffuse large B cell lymphomas of non-germinal centre type with respect to BCL2 gene aberrations.
Discussion
DLBCL forms a heterogeneous group of lymphoid neoplasms as reflected by its subdivision into several variants, subgroups and subtypes/entities in the most recent World Health Organization classification.1 Subgrouping of DLBCL by gene expression profiling has been shown to be of prognostic relevance.6 ,7 ,8 However, gene expression profiling cannot be applied in daily routine practice, necessitating a search for surrogate markers. One marker that merits particular attention is BCL2/bcl2, at the genetic level and the protein level, for a number of reasons: the prognostic importance of the bcl2 protein has been confirmed by numerous studies, the BCL2 gene is recurrently targeted by anomalies in 30–50% of DLBCLs, and bcl2-associated treatment resistance can be abolished by the addition of rituximab to CHOP-therapy regimens.11 ,12 ,13 ,17 ,18 ,19 ,20 ,22 ,28 ,29 ,34 ,35 ,36 ,37 ,38 ,39 ,40 ,41 ,42 ,43 ,44 ,45 The prognostic importance of BCL2/bcl2 in DLBCL remains a matter of intense debate. With few exceptions,45 there is no definitive evidence that the presence of a BCL2 translocation at diagnosis has any impact on the survival of patients with DLBCL, though the prognostic impact of bcl2 protein expression, evaluated in multiple large-scale trials, appears to be significant.11 ,17 ,19 ,43
In this high-throughput study on a standardised TMA platform, we were able to demonstrate that abnormalities, particularly gains, of the BCL2 gene are an IPI-independent negative prognostic factor for DSS in DLBCL of non-GC type. In addition, we could estimate the molecular epidemiology of BCL2 abnormalities within DLBCL with a focus on the phenotype. The detected incidence of 19% for breaks is in agreement with most previously published results.17 ,19 ,22 ,40 ,47 ,48 ,49 ,50 ,51 Though claimed to be exclusive to DLBCL of the GC type by some authors,46 other studies detected non-GC type DLBCL with BCL2 breaks,45 yet in slightly lower proportions than in our collective (5% versus 17%). Importantly in both previous analyses,45 ,46 IgH/BCL2 double-fusion probes, which can solely detect t(14;18) but not variant BCL2 translocations, were applied. In our study we used a BCL2 break-apart probe, which can identify various BCL2 translocations; this may explain the higher percentage of non-GC type DLBCLs with BCL2 breaks in our study. A correlation was found between gains and protein expression in our study that may be attributed to the gene-dosage effect. No correlation between breaks of the BCL2 gene and bcl2 protein expression was demonstrated, and this may be partly linked to mutations in the open reading frame of the translocated BCL2 gene causing lack of bcl2 protein expression despite a gene abnormality.52 We did not observe a prognostic relevance of bcl2 protein expression as opposed to reports previously published,11 ,13 ,17 ,18 ,19 ,20 ,34 ,35 ,36 ,37 but in keeping with others.22 ,38 ,53 This can be explained by a number of factors such as case mix of patients, different types of treatment (especially addition of rituximab), and different methodologies for cut-off level determination.
Considering our evidence of the different prognostic value of abnormalities of the BCL2 gene in GC and non-GC DLBLC and previous observations,7 ,20 ,22 ,28 ,29 ,30 it is tempting to speculate that these BCL2 abnormalities might play a different role in GC and non-GC DLBCL pathogenesis and progression. Translocations of BCL2 might be gate-keeping for DLBCLs of the GC type, but not those of the non-GC type, while in the latter group, structural and numeric BCL2 aberrations might represent additional genetic hits associated with tumour progression. In line with these speculations, in GC type DLBCL the mean proportion of cells with breaks exceeded that in non-GC DLBCL (59% versus 36%), while the opposite was true for gains (29% versus 35%). Within this model, where development of de novo DLBCL of the GC type might depend on BCL2 abnormalities, it is obvious that analysis of this gene will not contribute to prediction of its clinical course. On the other hand, BCL2 aberrations, particularly gains, in DLBCLs of the non-GC type might identify aggressive/progressed tumours. Of course, these considerations should be addressed in prospective large scale studies. Nevertheless, until precise identification of prognostically relevant subgroups/subtypes of DLBCL is accomplished, assessment of BCL2 gene abnormalities with respect to the lymphoma phenotype might aid in individual risk assessment in DLBCL.
Take-home messages
Diffuse large B cell lymphomas (DLBCLs) are characterised by a highly variable outcome, which to date cannot be predicted by biomarkers.
Breaks and gains of the BCL2 gene can easily be assessed by fluorescence in situ analysis (FISH).
Structural, and particularly numeric, abnormalities of BCL2 correlate with a significantly worse prognosis in DLBCL of non-germinal centre (non-GC) phenotype.
FISH analysis of the BCL2 gene may aid in the individual risk assessment of patients with DLBCL of non-GC type.
REFERENCES
Footnotes
Funding The study was partially supported by the Oncosuisse grant OCS-02072-04-2007.
Competing interests None.
Provenance and peer review Not commissioned; externally peer reviewed.